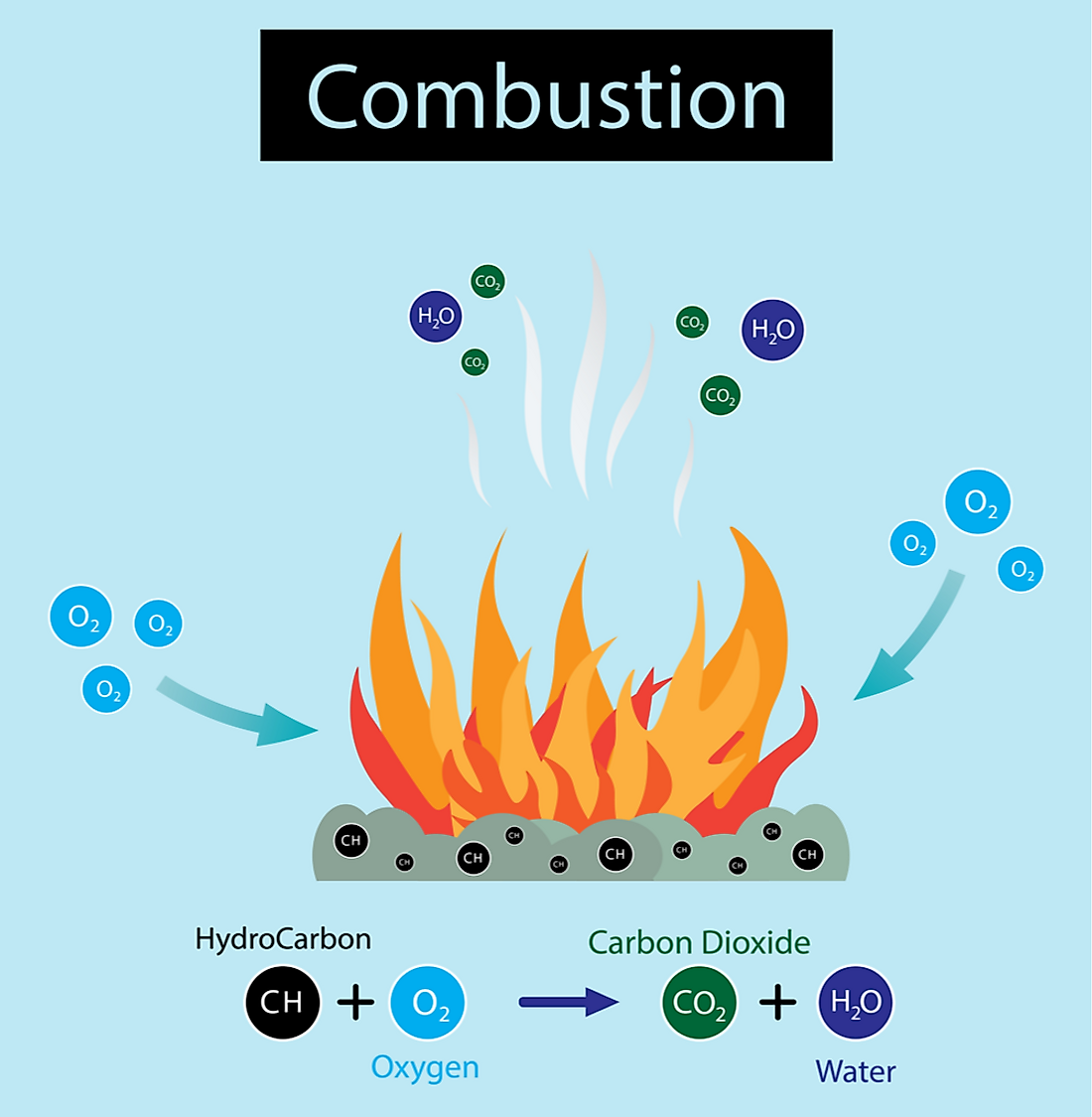
Two Types Of Combustion . Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Types of combustion include complete combustion, incomplete combustion,. Two types of hydrocarbon combustion have been defined: (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a chemical process that releases heat and light energy. Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product.
from www.worldatlas.com
Combustion is a chemical process that releases heat and light energy. Types of combustion include complete combustion, incomplete combustion,. Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. Two types of hydrocarbon combustion have been defined: (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product.
What Are The Properties Of Matter? WorldAtlas
Two Types Of Combustion Combustion is a chemical process that releases heat and light energy. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Combustion is a chemical process that releases heat and light energy. Types of combustion include complete combustion, incomplete combustion,. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. Two types of hydrocarbon combustion have been defined:
From gmbar.co
And Combustion Worksheet Free Download Gmbar.co Two Types Of Combustion In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. Combustion is a chemical process that releases heat and light energy. Types of combustion include. Two Types Of Combustion.
From www.thoughtco.com
Combustion Definition in Chemistry Two Types Of Combustion Two types of hydrocarbon combustion have been defined: Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. Types of combustion include complete combustion, incomplete combustion,. Combustion is a chemical process that releases heat and light energy. (1) slow combustion at temperatures below 500 °c, including. Two Types Of Combustion.
From www.worldatlas.com
What Are The Properties Of Matter? WorldAtlas Two Types Of Combustion Two types of hydrocarbon combustion have been defined: Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. (1) slow combustion at temperatures below 500. Two Types Of Combustion.
From mungfali.com
Different Types Of Combustion Two Types Of Combustion Two types of hydrocarbon combustion have been defined: In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. Combustion is a chemical process that releases. Two Types Of Combustion.
From www.ni.com
Subsystems Required to Control Low Temperature Combustion Engines Two Types Of Combustion (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a chemical process that releases heat and light energy. Two types of hydrocarbon combustion have been defined: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o),. Two Types Of Combustion.
From www.shmoop.com
Combustion Shmoop Two Types Of Combustion (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Two types of hydrocarbon combustion have been defined: Types of combustion include complete combustion, incomplete combustion,. Combustion is a reaction between a hydrocarbon fuel (e.g.,. Two Types Of Combustion.
From www.teachoo.com
Why are carbon and its compounds used as fuels for most applications? Two Types Of Combustion In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. Combustion is a chemical process that releases heat and light energy. (1) slow combustion at. Two Types Of Combustion.
From www.youtube.com
2 What is combustion? YouTube Two Types Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Two types of hydrocarbon combustion have been defined: Types of combustion include complete combustion, incomplete combustion,. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion. Two Types Of Combustion.
From www.slideserve.com
PPT COMBUSTION REACTIONS PowerPoint Presentation, free download ID Two Types Of Combustion (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a chemical process that releases heat and light energy. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Two types of hydrocarbon combustion have been defined: Combustion encompasses a great variety of. Two Types Of Combustion.
From www.slideserve.com
PPT 5 Types of Chemical Reactions PowerPoint Presentation, free Two Types Of Combustion Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. Types of combustion include complete combustion, incomplete combustion,. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a chemical process that releases heat and light energy. In the most. Two Types Of Combustion.
From www.researchgate.net
Prior studies categorized based on different types of internal Two Types Of Combustion Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. Combustion is a chemical process that releases heat and light energy. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane,. Two Types Of Combustion.
From www.youtube.com
Chemistry Types of combustion and flame English YouTube Two Types Of Combustion Types of combustion include complete combustion, incomplete combustion,. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion encompasses a great variety of phenomena with wide. Two Types Of Combustion.
From engineeringlearn.com
Types of Combustion Chamber Functions, Advantages & Disadvantages Two Types Of Combustion (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Two types of hydrocarbon combustion have been defined: Combustion is a chemical process that releases heat and light energy. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o),. Two Types Of Combustion.
From www.teachoo.com
Different Types of Combustion with Examples Teachoo Concepts Two Types Of Combustion Two types of hydrocarbon combustion have been defined: Types of combustion include complete combustion, incomplete combustion,. Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. Combustion is a chemical process that releases heat and light energy. (1) slow combustion at temperatures below 500 °c, including. Two Types Of Combustion.
From www.slideserve.com
PPT CHAPTER 6 COMBUSTION AND FLAME PowerPoint Presentation, free Two Types Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to. Two Types Of Combustion.
From vehq.com
The 5 Types Of Combustion Chambers To Know Two Types Of Combustion (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Two types of hydrocarbon combustion have been defined: Combustion is a chemical process that releases heat and light energy. Types of combustion include complete combustion,. Two Types Of Combustion.
From www.slideserve.com
PPT CHAPTER 6 COMBUSTION AND FLAME PowerPoint Presentation, free Two Types Of Combustion In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Types of combustion include complete combustion, incomplete combustion,. Two types of hydrocarbon combustion have been defined: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2),. Two Types Of Combustion.
From sciencenotes.org
Combustion Reaction Definition and Examples Two Types Of Combustion Combustion is a chemical process that releases heat and light energy. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Types of combustion include complete combustion, incomplete combustion,. Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on. Combustion is a. Two Types Of Combustion.